Justifying Advanced PAP Machines
Contents
Justifying ASV
ASV or Adaptive servo-ventilation (ASV)
Central Sleep Apnea (CSA) is the cessation of respiratory effort result in a lack of respiratory movements. During sleep, your breathing is disrupted regularly because of how your brain functions, your brain simply doesn't tell your body to breathe, and therefore you don't try to breathe.
- Apnea: 80% to 100% reduction in airflow for >= 10 seconds
- Hypopnea: 50% to 80% reduction in airflow for >= 10 seconds
- Flow Limitation: <50% reduction in airflow for >= 10 seconds
Five types of Central Sleep Apnea
per the Mayo Clinic Central sleep apnea occurs when your brain fails to transmit signals to your breathing muscles. Central sleep apnea can be caused by a number of conditions that affect the ability of your brainstem — which links your brain to your spinal cord and controls many functions such as heart rate and breathing — to control your breathing. The cause varies with the type of central sleep apnea you have.
Types include:
- Cheyne-Stokes breathing. This type of central sleep apnea is most commonly associated with congestive heart failure or stroke. This condition is characterized by a gradual increase and then decrease in breathing effort and airflow. During the weakest breathing effort, a total lack of airflow (central sleep apnea) can occur.
- Drug-induced apnea. Taking certain medications such as opioids — including morphine sulfate (Ms Contin, others), oxycodone (Oxycodone HCL, Oxycontin, others) or codeine sulfate — may cause your breathing to become irregular, to increase and decrease in a regular pattern, or to temporarily stop completely.
- High-altitude periodic breathing. A Cheyne-Stokes breathing pattern may occur if you're exposed to a very high altitude. The change in oxygen at this altitude is the reason for the alternating rapid breathing (hyperventilation) and underbreathing.
- Complex sleep apnea. Some people with obstructive sleep apnea develop central sleep apnea while using continuous positive airway pressure (CPAP) for their sleep apnea treatment. This condition is known as complex sleep apnea because it's a combination of obstructive and central sleep apneas.
- Medical condition-induced central sleep apnea. Several medical conditions may give rise to central sleep apnea of the non-Cheyne-Stokes variety.
- Idiopathic (primary) central sleep apnea. The cause of this uncommon type of central sleep apnea isn't known.
Diagnosis of Central Apnea
- Must have clinical symptoms to make the diagnosis - Sleepiness, insomnia, snoring, apneas, awakening with Shortness of Breath, A‐fib, CHF, or neurological disorder (such as stroke, MS)
- Central AHI >5
- Central apneas and Central hypopneas >50% of total AHI
- Not better explained by another sleep disorder
Hypopneas - Obstructive and Central
To determine the statistics for Central apneas and Central hypopneas >50% of total AHI above we need to determine which hypopneas are central in nature. The description below defines the difference between obstructive and central hypopneas.
An obstructive hypopnea contains one or more of the following:
- An increase in PAP flow signal
- Snoring during the event
- Paradoxical breathing
A central hypopnea will have none of the above.
Central Hypopnea. Central hypopneas are associated with reductions of purely in-phase thoracic and abdominal effort or movement signals, followed by an increase in chest and belly movements at the end. There is no evidence of phase shifting or paradoxical breathing, no airflow flattening, and no snoring throughout the entire central hypopnea.
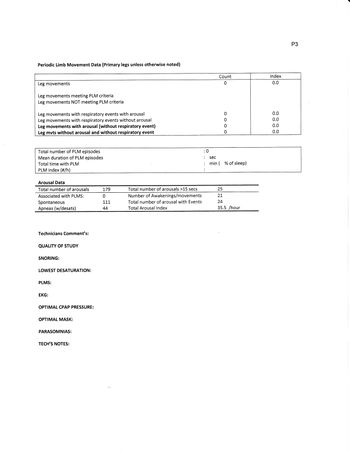
A Central AHI is composed of Central Apnea and Central Hypopnea. The Central Apnea numbers are easily extracted from modern PAP machines which report detailed efficacy data. We need to concentrate on Central Hypopnea numbers to demonstrate a Central AHI >5 and that Central apneas and Central hypopneas >50% of total AHI
Central Hypopnea. Central hypopneas are associated with reductions of purely in-phase thoracic and abdominal effort or movement signals, followed by an increase in chest and belly movements at the end. There is no evidence of phase shifting or paradoxical breathing, no airflow flattening, and no snoring throughout the entire central hypopnea.
Paradoxical Breathing explanation: The chest and abdomen should expand when they inhale and contract when they exhale. If the chest and abdomen contract while inhaling and expand while breathing out, a person may have paradoxical breathing.
Charts identifying Central Hypopneas
Failed Sleep Studies
Demonstrations of significant Central Apneas at Trial pressures
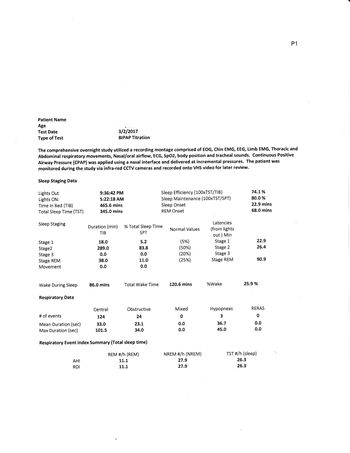
Although the conclusion of your study is severe obstructive apnea, your diagnostic study before PAP shows predominately central apnea, but enough OA that this would lead to a likely diagnosis of complex or mixed apnea in the severe range. Central events were more numerous and longer in duration than obstructive, with an event actually going more than 1-1/2 minutes. Bet you couldn't hold your breath that long if your were awake! Your BiPAP titration did reduce OA and helped significantly with your sleep architecture and some of the effects of apnea such as snoring; however, the report concedes you continue to have CA. The CA "improved" with final pressure but was not resolved.
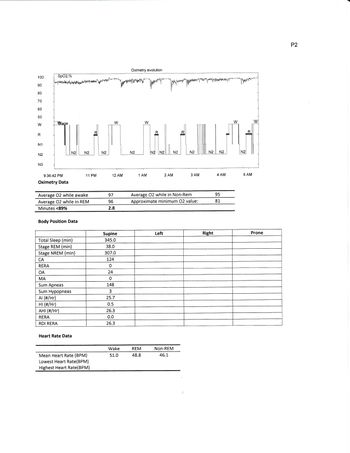
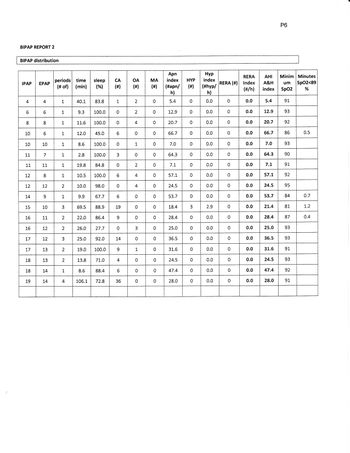
A glance at the charts on page 4 shows that at the beginning of the night you had predominately OA events. You seemed to do pretty good at CPAP pressure of 11/11 for about 15 minutes during N2 sleep. They moved you to 16/12 bipap during a wake period that transitioned to N2 sleep. They then dropped pressure to 10/6 and you immediately had abundant CA, which transitioned to OA and back to CA as bipap pressure increased. A 15 minute period at 14/9 just after 1:00 had no apnea, but you were recorded as awake. Pressure increased to 15/10 then 19/14 with a brief drop-out of events. It appears that this period may be the basis of your titrated BiPAP pressure.
My impression is that you are headed for ASV (hopefully soon). This titration shows you were not successfully treated at any of the pressures attempted, and no trial lasted longer than 15 minutes. Your BiPAP titration is the best compromise found, with none being in a range that would be considered effective. Your results since the titration demonstrate that you will not succeed at bilevel without a backup to treat centrals, and your treated complex apnea remains in the high moderate to severe range. The titration study tells me, that there is not an effective pressure range any of us can recommend to you that will result in an acceptable AHI for any long-term.
I'm sorry to say, there is nothing I can suggest to improve your results other than you need to be sure your doctor is aware that you continue to have severe mixed sleep apnea, with many central and obstructive events, and for you to push to obtain ASV at the earliest possible time.
Sleep Study on BiPAP determined events rated according to the machine data remains very high with mixed events. The machine data suggest the results are much worse than the untreated AHI. You should tell them you have looked at the results of the tests and don't really see how it was determined (1) that you have obstructive apnea, and (2), how it was determined that you had a "good response" to bipap pressure of 15/10. At 1:15 at 14/9 and 15/10 shows central abundant apnea (approximately 30 AHI to numerous to count). At 1:45 to 2:15 15/10 was tried for 30 minutes with 11 apnea (AHI 22) with the longest apnea of the night at 2:00 at 15/10. A final period of 15/10 was tried at about 4:30, lasting about 15 minutes (deducting for wake) with 2 OA events (AHI 8) No sustained period of efficacy (ahi<5) was found. Your ongoing machine data confirm any efficacy found during the titration study may have been coincidental or an anomaly. You should flatly say in your opinion BiPAP at the prescribed pressures is not working, and explain any symptoms you feel (fatigue, frequent awakenings, whatever).
CSA-and-ASV-Updated-Morgan.pdf
Requirements for Medicare
Respiratory Assist Device (RAD)
Order Template Guidance
Purpose
This template is designed to assist a clinician in completing a Written Order Prior to Delivery (WOPD) in order for a Respiratory Assist Device (RAD) and Detailed Written Order (DWO) for accessories to meet requirements for Medicare eligibility and coverage. When completed appropriately, this template meets requirements for a WOPD or a DWO. The clinician can keep the completed template on file within the patient’s medical record or it can be used to develop an order template for use with the system containing the patient’s electronic medical record.
Patient eligibility for coverage of RAD therapy under Medicare Eligibility for coverage of RAD therapy and accessories under Medicare requires a physician or qualified Non-Physician Practitioner (NPP, nurse practitioner, clinical nurse specialist, or physician assistant) to establish that coverage criteria are met. This helps to ensure the RAD and accessories provided are consistent with the physician’s prescription and supported in the patient’s medical record.
A Face-to-Face (F2F) encounter is required by Medicare for the following RAD devices:
- E0470 - RESPIRATORY ASSIST DEVICE, BI-LEVEL PRESSURE CAPABILITY, WITHOUT BACKUP RATE FEATURE, USED WITH NONINVASIVE INTERFACE, E.G., NASAL OR FACIAL MASK (INTERMITTENT ASSIST DEVICE WITH CONTINUOUS POSITIVE AIRWAY PRESSURE DEVICE)
- E0471 - RESPIRATORY ASSIST DEVICE, BI-LEVEL PRESSURE CAPABILITY, WITH BACK-UP RATE FEATURE, USED WITH NONINVASIVE INTERFACE, E.G., NASAL OR FACIAL MASK (INTERMITTENT ASSIST DEVICE WITH CONTINUOUS POSITIVE AiRWAY PRESSURE DEVICE)
NOTE: RAD accessories do not require a F2F encounter, but do require a DWO.
The F2F Encounter must be completed within a 6-month timeframe prior to completion of the WOPD that starts RAD therapy for the treatment of a clinical condition supported by the patient’s diagnosis.
Indications for use of a RAD is divided into the following categories:
- Restrictive thoracic disorders, (i.e., progressive neuromuscular disorders or severe thoracic cage abnormalities);
- Severe chronic obstructive pulmonary disease (COPD); use of a RAD in COPD patients requires,
- A facility-based polysomnogram to rule out obstructive sleep apnea in order to initiate Medicare coverage,
- A prerequisite trial of noninvasive ventilation without a backup rate, and
- Treatment with continuous positive airway pressure devices.
- Central sleep apnea, i.e., apnea not due to airway obstruction;
- Hypoventilation; and
- Obstructive sleep apnea (OSA).
See Appendices A&B for further guidance on indications for use and coverage.
Initial coverage -- first three (3) months of therapy The medical record must document symptoms characteristic of sleep-associated hypoventilation, e.g.:
- Daytime hypersomnolence;
- Excessive fatigue;
- Morning headache;
- Cognitive dysfunction;
- Dyspnea, etc.; and
- Beneficiary has one (1) of the disorders listed above and meets all coverage criteria for that disorder as listed below.
Continued coverage (beyond the first three months of therapy) - E0470 or E0471 Medical record documentation has a signed and dated statement that the beneficiary was re-evaluated on/after the 61st day of therapy demonstrating:
- Progress of relevant symptoms; and
- Beneficiary usage of the device (average 4 hours per 24 hours)
Beneficiaries entering Medicare There must be documentation of the following:
- A qualifying test confirming that the beneficiary had testing prior to Fee-for-Service (FFS)
Medicare enrollment, that meets the current coverage criteria in effect at the time that the beneficiary seeks Medicare coverage of a replacement device and/or accessory; and
- There must be a F2F clinical evaluation following enrollment in FFS Medicare that confirms all of the following:
- The beneficiary has the qualifying medical condition for the applicable scenario; and
- Testing performed, date of the testing used for qualification and results; and
- The beneficiary continues to use the device; and
- The beneficiary is benefiting from the treatment.
Other guidance
Completing the RAD order template does not guarantee eligibility and coverage but does provide an area within the patient’s medical file that is readily identifiable and available in support of RAD therapy equipment and accessories ordered and billed to Medicare. This template may be used with the Respiratory Assist Device Laboratory Test Results Template and Respiratory Assist Device Face to Face Template.
Who can complete the Respiratory Assist Device Order Template? Physician/NPP who performs the F2F Encounter
File:Respiratory-Assist-Device-Order-Template-Draft-20180412-R10b.pdf

Donate to Apnea Board